当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu2O nanoparticles with controlled surface roughness for CO2 electroreduction towards C2+ products
Chemical Engineering Journal ( IF 13.2 ) Pub Date : 2025-05-01 , DOI: 10.1016/j.cej.2025.163293 Qi Fang , Zining Zhang , Xue Yang , Tao Cheng , Xinyan Ma , Jing Tang
Chemical Engineering Journal ( IF 13.2 ) Pub Date : 2025-05-01 , DOI: 10.1016/j.cej.2025.163293 Qi Fang , Zining Zhang , Xue Yang , Tao Cheng , Xinyan Ma , Jing Tang
|
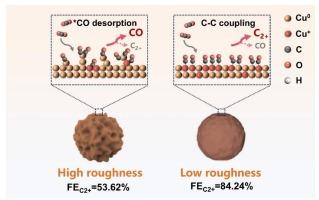
Electrocatalytic CO2 reduction to produce multi-carbon (C2+ ) products represents a promising avenue for carbon recycling and CO2 conversion industrialization. However, improving C2+ selectivity remains challenging due to uncontrollable reaction pathways. Adjusting the surface properties of the catalyst can effectively influence the nature of the active sites and the branching of the reaction pathways. Herein, we successfully synthesized of Cu2 O catalysts with different roughness by adjusting the amount of surfactant, a structure-directing agent. The optimal catalyst indicated a remarkable FEC2+ of 84.2 % at −1.4 V vs . RHE, with an ethylene selectivity of 56.3 % and C2+ partial current density of 416.4 mA/cm2 in a flow cell. In-situ spectroscopy characterization and COMSOL simulations revealed that *CO intermediates tend to desorb from the rough surface, which accelerates the reduction of active sites Cu+ and leads to CO formation. In contrast, flat catalyst surfaces facilitate the carbon intermediate adsorption, thereby stabilizing Cu+ and promoting C-C coupling, ultimately enhancing C2+ production. This work not only refines the structure–activity relationship between catalyst surface roughness and products selectivity, but also offers strategies for designing efficient catalysts from the perspective of surface properties.
中文翻译:

具有受控表面粗糙度的 Cu2O 纳米颗粒,用于 CO2 电还原到 C2+ 产品
电催化 CO2 还原生产多碳 (C2+) 产品代表了碳回收和 CO2 转化工业化的一条有前途的途径。然而,由于反应途径不可控,提高 C2+ 选择性仍然具有挑战性。调整催化剂的表面性质可以有效影响活性位点的性质和反应途径的分支。在此,我们通过调整结构导向剂表面活性剂的用量,成功合成了具有不同粗糙度的 Cu2O 催化剂。最佳催化剂表明,与 RHE 相比,在 -1.4 V 下,FEC2+ 显著为 84.2 %,乙烯选择性为 56.3 %,C2+ 部分电流密度为 416.4 mA/cm2。原位光谱表征和 COMSOL 仿真表明,*CO 中间体倾向于从粗糙表面解吸,这加速了活性位点 Cu+ 的还原并导致 CO 形成。相反,平坦的催化剂表面有利于碳中间体的吸附,从而稳定 Cu+ 并促进 C-C 耦合,最终增强 C2+ 的产生。这项工作不仅细化了催化剂表面粗糙度与产物选择性之间的构效关系,而且从表面性质的角度为设计高效催化剂提供了策略。
更新日期:2025-05-01
中文翻译:

具有受控表面粗糙度的 Cu2O 纳米颗粒,用于 CO2 电还原到 C2+ 产品
电催化 CO2 还原生产多碳 (C2+) 产品代表了碳回收和 CO2 转化工业化的一条有前途的途径。然而,由于反应途径不可控,提高 C2+ 选择性仍然具有挑战性。调整催化剂的表面性质可以有效影响活性位点的性质和反应途径的分支。在此,我们通过调整结构导向剂表面活性剂的用量,成功合成了具有不同粗糙度的 Cu2O 催化剂。最佳催化剂表明,与 RHE 相比,在 -1.4 V 下,FEC2+ 显著为 84.2 %,乙烯选择性为 56.3 %,C2+ 部分电流密度为 416.4 mA/cm2。原位光谱表征和 COMSOL 仿真表明,*CO 中间体倾向于从粗糙表面解吸,这加速了活性位点 Cu+ 的还原并导致 CO 形成。相反,平坦的催化剂表面有利于碳中间体的吸附,从而稳定 Cu+ 并促进 C-C 耦合,最终增强 C2+ 的产生。这项工作不仅细化了催化剂表面粗糙度与产物选择性之间的构效关系,而且从表面性质的角度为设计高效催化剂提供了策略。






























































 京公网安备 11010802027423号
京公网安备 11010802027423号