当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Switching CO2 Electroreduction toward C2+ Products and CH4 by Regulating the Dimerization and Protonation in Platinum/Copper Catalysts
Angewandte Chemie International Edition ( IF 16.9 ) Pub Date : 2025-01-23 , DOI: 10.1002/anie.202424749 Tailei Hou 1 , Jiexin Zhu 2 , Hongfei Gu 1 , Xinyuan Li 1 , Yiqing Sun 3 , Ze Hua 1 , Ruiwen Shao 1 , Cheng Chen 4 , Botao Hu 5 , Liqiang Mai 2 , Shenghua Chen 3 , Dingsheng Wang 6 , Jiatao Zhang 1
Angewandte Chemie International Edition ( IF 16.9 ) Pub Date : 2025-01-23 , DOI: 10.1002/anie.202424749 Tailei Hou 1 , Jiexin Zhu 2 , Hongfei Gu 1 , Xinyuan Li 1 , Yiqing Sun 3 , Ze Hua 1 , Ruiwen Shao 1 , Cheng Chen 4 , Botao Hu 5 , Liqiang Mai 2 , Shenghua Chen 3 , Dingsheng Wang 6 , Jiatao Zhang 1
Affiliation
|
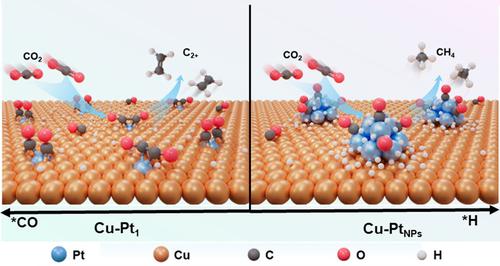
Copper (Cu)‐based catalysts exhibit distinctive performance in the electrochemical CO2 reduction reaction (CO2 RR) with complex mechanism and sophisticated types of products. The management of key intermediates *CO and *H is a necessary factor for achieving high product selectivity, but lack of efficient and versatile strategies. Herein, we designed Pt modified Cu catalysts to effectively modulate the competitive coverage of those intermediates. The Pt single‐atoms and Pt nanoparticles modified Cu catalysts (denoted as Cu‐Pt1 and Cu‐PtNPs ) precisely regulated the protonation and dimerization, with the faradaic efficiency (FE) of C2+ products up to 70.4 % and the FE of CH4 reaching 57.7 %, respectively. CO stripping experiments reveal that Pt1 sites could enhance the adsorption of *CO, while PtNPs exhibit *CO tolerance for H2 O dissociation. In situ spectroscopic results further confirms that high coverage of *CO is achieved on Cu‐Pt1 , while *CHO on Cu‐PtNPs might generate by additional water dissociation. As elucidated by theoretical studies, the interfacial sites of Cu‐Pt1 would favor the *CO coverage promoting the evolution of *OCCO for C2+ products while PtNPs supplementarily accelerate H2 O dissociation achieving *CHO for CH4 . This work provides insights for efficient and targeted CO2 conversion by atomically design of active sites with engineered key intermediates coverage.
中文翻译:

通过调节铂/铜催化剂中的二聚化和质子化,将 CO2 电还原转向 C2+ 产物和 CH4
铜 (Cu) 基催化剂在电化学 CO2 还原反应 (CO2RR) 中表现出独特的性能,具有复杂的机理和复杂的产物类型。关键中间体 *CO 和 *H 的管理是实现高产品选择性的必要因素,但缺乏高效和通用的策略。在此,我们设计了 Pt 改性 Cu 催化剂,以有效调节这些中间体的竞争覆盖率。Pt 单原子和 Pt 纳米颗粒修饰的 Cu 催化剂 (表示为 Cu-Pt1 和 Cu-PtNPs) 精确调节质子化和二聚化,C2+ 产物的法拉第效率 (FE) 高达 70.4 %,CH4 的法拉第效率 (FE) 分别达到 57.7 %。CO 剥离实验表明,Pt1 位点可以增强 *CO 的吸附,而 PtNPs 对 H2O 解离表现出 *CO 耐受性。原位光谱结果进一步证实,在 Cu-Pt1 上实现了 *CO 的高覆盖率,而 Cu-PtNP 上的 *CHO 可能通过额外的水解离产生。正如理论研究所阐明的那样,Cu-Pt1 的界面位点将有利于 *CO 覆盖率,促进 C2+ 产物的 *OCCO 的进化,而 PtNPs 补充加速 H2O 解离,实现 CH4 的 *CHO。这项工作通过原子设计具有工程关键中间体覆盖率的活性位点,为高效和有针对性的 CO2 转化提供了见解。
更新日期:2025-01-23
中文翻译:

通过调节铂/铜催化剂中的二聚化和质子化,将 CO2 电还原转向 C2+ 产物和 CH4
铜 (Cu) 基催化剂在电化学 CO2 还原反应 (CO2RR) 中表现出独特的性能,具有复杂的机理和复杂的产物类型。关键中间体 *CO 和 *H 的管理是实现高产品选择性的必要因素,但缺乏高效和通用的策略。在此,我们设计了 Pt 改性 Cu 催化剂,以有效调节这些中间体的竞争覆盖率。Pt 单原子和 Pt 纳米颗粒修饰的 Cu 催化剂 (表示为 Cu-Pt1 和 Cu-PtNPs) 精确调节质子化和二聚化,C2+ 产物的法拉第效率 (FE) 高达 70.4 %,CH4 的法拉第效率 (FE) 分别达到 57.7 %。CO 剥离实验表明,Pt1 位点可以增强 *CO 的吸附,而 PtNPs 对 H2O 解离表现出 *CO 耐受性。原位光谱结果进一步证实,在 Cu-Pt1 上实现了 *CO 的高覆盖率,而 Cu-PtNP 上的 *CHO 可能通过额外的水解离产生。正如理论研究所阐明的那样,Cu-Pt1 的界面位点将有利于 *CO 覆盖率,促进 C2+ 产物的 *OCCO 的进化,而 PtNPs 补充加速 H2O 解离,实现 CH4 的 *CHO。这项工作通过原子设计具有工程关键中间体覆盖率的活性位点,为高效和有针对性的 CO2 转化提供了见解。






























































 京公网安备 11010802027423号
京公网安备 11010802027423号