当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhancing CO2 Electroreduction to Multicarbon Products by Modulating the Surface Microenvironment of Electrode with Polyethylene Glycol
Angewandte Chemie International Edition ( IF 16.9 ) Pub Date : 2025-01-08 , DOI: 10.1002/anie.202420661 Yiyong Wang 1, 2 , Yingying Cheng 1 , Shiqiang Liu 1 , Yaoyu Yin 1, 2 , Jiahao Yang 1, 2 , Hengan Wang 1, 2 , Ke Li 1, 2 , Meng Zhou 1 , Jiapeng Jiao 3 , Pei Zhang 1 , Qingli Qian 1, 2 , Qinggong Zhu 1, 2 , Xiaofu Sun 1, 2 , Yi Xu 1, 2 , Mingchuan Luo 4 , Xinchen Kang 1, 2 , Buxing Han 1, 2, 3
Angewandte Chemie International Edition ( IF 16.9 ) Pub Date : 2025-01-08 , DOI: 10.1002/anie.202420661 Yiyong Wang 1, 2 , Yingying Cheng 1 , Shiqiang Liu 1 , Yaoyu Yin 1, 2 , Jiahao Yang 1, 2 , Hengan Wang 1, 2 , Ke Li 1, 2 , Meng Zhou 1 , Jiapeng Jiao 3 , Pei Zhang 1 , Qingli Qian 1, 2 , Qinggong Zhu 1, 2 , Xiaofu Sun 1, 2 , Yi Xu 1, 2 , Mingchuan Luo 4 , Xinchen Kang 1, 2 , Buxing Han 1, 2, 3
Affiliation
|
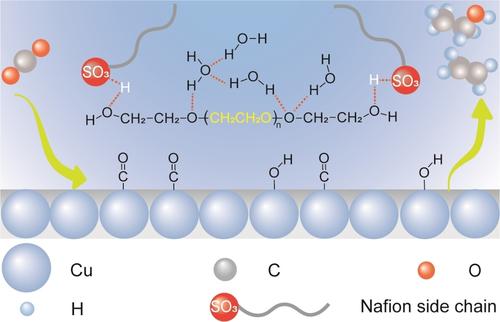
Modulating the surface microenvironment of electrodes stands as a pivotal aspect in enhancing the electrocatalytic performance for CO2 electroreduction. Herein, we propose an innovative approach by incorporating a small amount of linear oligomer, polyethylene glycol (PEG), into Cu2 O catalysts during the preparation of the CuPEG electrode. The Faradaic efficiency (FE) toward multicarbon products (C2+ ) increases from 69.3 % over Cu electrode without PEG to 90.3 % over CuPEG electrode at 500 mA cm−2 in 1 M KOH in a flow cell. In situ investigations and theoretical calculations reveal that PEG molecules significantly modify the microenvironment on the Cu surface through hydrogen bond interactions. This modification leads to the relaxation of Nafion, increasing the availability of active sites and enhancing the adsorption of *CO and *OH, which in turn promotes C−C coupling. Concurrently, the reconstructed hydrogen bond network reduces the presence of active hydrogen species, thereby inhibiting the hydrogen evolution reaction.
中文翻译:

通过用聚乙二醇调节电极的表面微环境来增强 CO2 电还原为多碳产物
调节电极的表面微环境是提高 CO2 电还原电催化性能的关键方面。在此,我们提出了一种创新方法,在制备 CuPEG 电极期间将少量线性低聚物聚乙二醇 (PEG) 掺入 Cu2O 催化剂中。在流通池中,在 1 M KOH 中,在 500 mA cm-2 下,对多碳产物 (C2+) 的法拉第效率 (FE) 从不含 PEG 的 Cu 电极的 69.3% 增加到 CuPEG 电极的 90.3%。原位研究和理论计算表明,PEG 分子通过氢键相互作用显着改变了 Cu 表面的微环境。这种修饰导致 Nafion 松弛,增加活性位点的可用性并增强 *CO 和 *OH 的吸附,进而促进 C-C 偶联。同时,重建的氢键网络减少了活性氢种类的存在,从而抑制了析氢反应。
更新日期:2025-01-08
中文翻译:

通过用聚乙二醇调节电极的表面微环境来增强 CO2 电还原为多碳产物
调节电极的表面微环境是提高 CO2 电还原电催化性能的关键方面。在此,我们提出了一种创新方法,在制备 CuPEG 电极期间将少量线性低聚物聚乙二醇 (PEG) 掺入 Cu2O 催化剂中。在流通池中,在 1 M KOH 中,在 500 mA cm-2 下,对多碳产物 (C2+) 的法拉第效率 (FE) 从不含 PEG 的 Cu 电极的 69.3% 增加到 CuPEG 电极的 90.3%。原位研究和理论计算表明,PEG 分子通过氢键相互作用显着改变了 Cu 表面的微环境。这种修饰导致 Nafion 松弛,增加活性位点的可用性并增强 *CO 和 *OH 的吸附,进而促进 C-C 偶联。同时,重建的氢键网络减少了活性氢种类的存在,从而抑制了析氢反应。






























































 京公网安备 11010802027423号
京公网安备 11010802027423号